Abstract
Introduction: A majority of AML patients ultimately relapse after achieving remission or exhibit refractory disease. Some patients, e.g., those carrying FLT3 -ITD mutation or other risk factors, may relapse early, i.e., within 6 months of initial diagnosis. Limited data exist regarding the economic impact of R/R AML. The present study was conducted to assess the economic burden associated with R/R AML, including early relapse, among commercially insured US patients.
Methods: Adults (≥18 years) with ≥2 independent claims of new AML diagnosis code not in remission or relapse (ICD-9-CM: 205.00) were identified from 2006-2016 in the QuintilesIMS Real-World Data Adjudicated Claims - US representing a large commercially insured US population. Included patients had continuous enrollment in a health plan for ≥6 months prior to and at ≥3 months after a new AML diagnosis and were longitudinally followed up until end of continuous enrollment or end of follow-up. Patients were categorized as R/R or non-R/R AML during the follow-up period, based on a validated algorithm (Earle et al, Med Care . 2002;40(8), IV-75), augmented by including ICD-9/-10 AML relapse diagnosis codes. R/R patients were further stratified as rapid progressors (RP) if the R/R event occurred within 6 months of first AML diagnosis. Healthcare resource utilization (HCRU) and costs were compared between: (1) R/R and non-R/R cohorts, and (2) RP and non-RP groups within the R/R cohort. HCRU was compared using adjusted incidence rate ratios (IRR) derived from multivariate generalized linear models (GLM) controlling for baseline demographic characteristics (age, sex, region, payer type) and Charlson comorbidity index (CCI), and was reported as the likelihood of using a defined healthcare resource over a patient month. The burden of total cost and its components was compared using mean total per patient per month (PPPM) healthcare costs to account for variable follow-up. The adjusted cost differences were derived from separate GLMs.
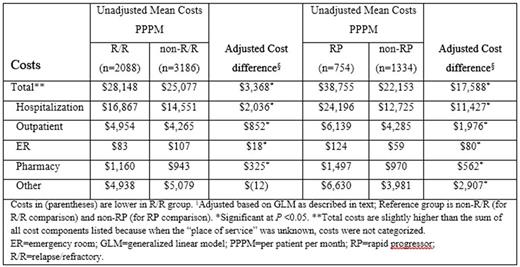
Results: 5,274 newly diagnosed AML patients were included. Median age was 58 years, 52% were male, 12% had CCI ≥3, and median follow-up time was 16 months. Forty percent (2,088 out of 5,274) had R/R AML. Among R/R patients, the median (95% CI) time from AML diagnosis to R/R was 7.5 (7.4-7.8) months. Compared to non-R/R patients, R/R patients had 71% more transfusions (IRR: 1.71 [95% CI: 1.53-1.91]), 45% more hospitalizations (IRR: 1.45 [95% CI: 1.29-1.62]), 14% longer median days in hospital (IRR: 1.14 [95% CI: 1.05-1.24], 87% more outpatient visits (IRR: 1.87 [95% CI: 1.73-2.03]), and almost 3 times more hospice admissions (IRR: 2.70 [95% CI: 1.40-5.19]); all P <0.001. ER admissions were not significantly different (IRR: 1.19 [95% CI: 0.87-1.61]), P= 0.278. Unadjusted PPPM total costs were significantly higher for R/R vs non-R/R patients ($28,148 vs $25,077; adjusted difference of $3,368; P <0.001), with the main driver being hospitalizations ($16,867 vs $14,551; adjusted difference of $2,036; P <0.001). In the R/R cohort, RP patients had 44% more transfusions (IRR: 1.44 [95% CI: 1.23-1.68]), 62% more hospitalizations (IRR: 1.62 [95% CI: 1.37-1.92]), 92% longer median days in hospital (IRR: 1.92 [95% CI: 1.73-2.14]), 32% more outpatient visits (IRR: 1.32 [95% CI: 1.18-1.48]), 86% more ER admissions (IRR: 1.86 [95% CI: 1.16-2.99]), and over 3 times more hospice admissions (IRR: 3.11 [95% CI: 1.35-7.17]); all P <0.001, than non-RP patients. Unadjusted PPPM total costs were significantly higher in the RP versus non-RP patients ($38,755 vs $22,153; adjusted difference of $17,588; P <0.001), with the main driver being hospitalizations ($24,196 vs $12,725; adjusted difference of $11,427; P <0.001). See Table for cost components comparison.
Conclusions: Compared to the non-R/R AML patients, the R/R patients experienced significantly higher monthly healthcare resources and costs. This economic burden was particularly high among those progressing early. Hospitalization was the key driver of this increased burden. Delaying relapse in rapidly progressing patients may reduce the burden to the health care system.
Aly: Pharmerit International: Employment; Daiichi Sankyo, Inc.: Other: Grant; BMS: Other: Grant; Celgene: Other: Grant; Amgen: Other: Grant; AstraZeneca: Other: Grant; Pharmacyclics: Other: Grant; Celldex Therapeutics: Other: Grant. Bapat: Daiichi Sankyo, Inc.: Other: Grant; Pharmerit International: Employment. Ray: Daiichi Sankyo, Inc.: Employment, Equity Ownership. Chen: Pharmerit International: Employment; Daiichi Sankyo, Inc.: Other: Grant. Botteman: Daiichi Sankyo, Inc.: Other: Grant; Pharmerit International: Employment.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal